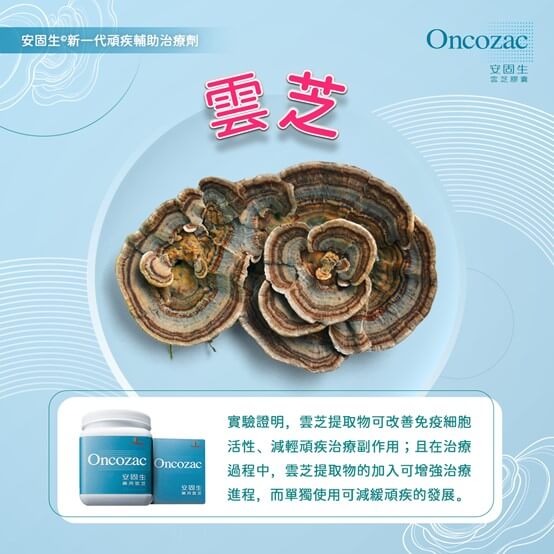
Live life the way you're meant to — Promoting a better Quality of Life with Oncozac®
Co-developed with the School of Pharmacy at the Chinese University of Hong Kong and containing the United States Pharmacopeia-verified ingredient ONCO-Z®, Oncozac® is proven to relieve treatment-induced side effects (such as nausea and fatigue) to promote a better quality of life25, 29, and ultimately increase survival rates for serious illness patients7,11,14,15,18,27.








Up to 6x more effective than similar products8
Experiments have revealed that Oncozac® is up to 6 times more effective in stimulating the proliferation of immune cells than similar products on the market. In immunological tests, an optimal dosage of Oncozac® for 7 consecutive days is shown to boost immune cells and in turn reduce the risk of infection8.


Certification 1st Chinese medicine ingredient verified by the United States Pharmacopeial (USP)*
- The ingredients meet label or certificate of analysis claims for identity, strength, purity, and quality
- The ingredients are consistent in quality from batch to batch
- The ingredients are prepared in accordance with accepted manufacturing practices
- In addition to USP-verification, Oncozac® also complies with GMP and SGS standards*Oncozac®'s only ingredient ONCO-Z® is the dietary ingredient verified by the United States Pharmacopeial Convention
Research & innovation Continuous research and experimentation
For over two decades, we have been collaborating with leading research institutions to conduct extensive tests studies on the efficacy of Oncozac® in treating serious illnesses.
Collaborations with leading research institutions worldwide
Oncozac® has been researched and developed with the Chinese University of Hong Kong since 1997.
Purapharm® has also worked with various public and private research institutions to support Oncozac®'s efficacy and safety.
Continued R&D investments for further clinical research
We continue to invest in Research & Development while working with leading research institutions both in Hong Kong and abroad. As a next step, we are working towards clinical studies for Triple-Negative Breast-related serious illness patients.